Lithium Polymer (LiPo) batteries is rechargeable lithium-ion battery that uses a polymer electrolyte instead of a liquid .
This design offers several advantages in terms of weight, form factor, and safety, making LiPo batteries popular in various applications, especially in consumer electronics and hobbyist drones. Here’s an overview:
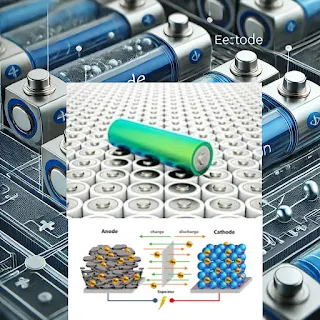
1.Basic Structure and Operation:
Electrodes:
Anode (Negative Electrode):
Typically made of graphite.
Cathode (Positive Electrode):
Usually composed of lithium metal oxide, such as lithium cobalt oxide (LiCoO₂), lithium iron phosphate (LiFePO₄), or other variants.
Electrolyte:
Unlike traditional lithium-ion batteries, which use a liquid electrolyte, LiPo batteries use a solid or gel-like polymer electrolyte, which can be safer and more flexible.
Separator:
A porous membrane between the anode and cathode that allows lithium ions to pass through while preventing direct contact between the electrodes, which could cause a short circuit.
2. Advantages
-Lightweight and Flexible Form Factor:
LiPo batteries can be made very thin and light, and they can be shaped to fit into compact spaces, which is particularly advantageous in devices where space and weight are critical, such as drones, smartphones, and laptops.
High Energy Density:
LiPo batteries offer a high energy density, meaning they can store a lot of energy relative to their weight and size.
Low Self-Discharge Rate:
LiPo batteries have a relatively low self-discharge rate, which means they can hold their charge for longer periods when not in use.
High Discharge Rate:
LiPo batteries can provide high currents, which is ideal for applications that require rapid bursts of power, such as in RC vehicles and drones.
3. Challenges
Safety Concrns:
While safer than some other lithium-ion chemistries, LiPo batteries can still be prone to swelling, overheating, and, in extreme cases, catching fire if not handled correctly. Damage, overcharging, or puncturing the battery can lead to dangerous situations.
Shorter Lifespan:
Compared to other battery chemistries, LiPo batteries typically have a shorter lifespan, often lasting around 300-500 charge cycles before significant capacity degradation occurs.
Cost.
iPo batteries can be more expensive than other types of batteries due to their advanced materials and manufacturing processes.
Sensitivity to Overcharging/Deep Discharging:
LiPo batteries require careful charging and discharging management. Overcharging or deeply discharging a LiPo battery can lead to irreversible damage or safety hazards.
4. Applications
Consumer Electronics:
Due to their lightweight and thin profile, LiPo batteries are commonly used in smartphones, tablets, laptops, and wearable devices.
RC Models and Drones:
LiPo batteries are the preferred choice for hobbyist RC cars, airplanes, helicopters, and drones, where high power output and lightweight are crucial.
Portable Power Banks:
Many high-capacity power banks use LiPo batteries for their superior energy density and slim form factor.
Electric Vehicles:
Although less common than other battery types like lithium-ion cylindrical cells, LiPo batteries are sometimes used in electric vehicles, especially in smaller or specialized models.
5. Maintenance and Safety Tips
Proper Charging:
Always use a charger designed specifically for LiPo batteries, which will typically include features to balance the cells and prevent overcharging.
Avoid Deep Discharge:
Try not to discharge a LiPo battery below 3.0 volts per cell, as this can significantly shorten its lifespan or even render the battery unusable.
Safe Storage:
Store LiPo batteries in a cool, dry place at around 50% charge if you won’t be using them for an extended period. This reduces stress on the battery and helps maintain its lifespan.
Monitor Temperature:
Avoid exposing LiPo batteries to high temperatures, as this can lead to swelling or other safety issues.
6. Future Prospects
Improved Safety:
Research is ongoing to develop safer LiPo batteries, including advancements in solid-state electrolytes and more robust battery management systems (BMS).
Higher Energy Densities:
Continued innovations aim to increase the energy density of LiPo batteries, making them even more suitable for demanding applications like electric vehicles and drones.
Cost Reduction:
As manufacturing processes improve and materials become more cost-effective, the price of LiPo batteries is expected to decrease, making them more accessible for a wider range of applications.
LiPo batteries are a crucial component in modern portable electronics and high-performance applications, offering a blend of lightweight design, high energy density, and flexibility. However, they require careful handling to ensure safety and longevity.







.png)


.jpeg)

.jpeg)






.webp)